AI From Theory to Clinics
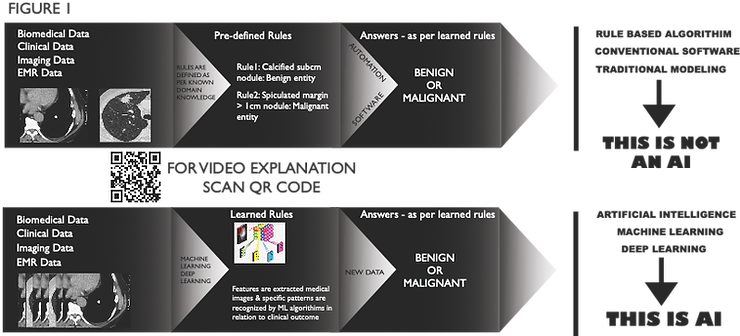
Artificial intelligence (AI) is a reality seen in many apps and devices. It has made inroads into almost all the domains of life. To understand AI, we first must know that it’s much more than automation, which is not AI.
Creating rules on historical data and then predicting the outcome is not an AI; it’s automation or advanced statistics. When laws are themselves figured out by algorithm when they are fed with historical data and their outcome, then it’s true AI.
Healthcare Data
To make AI algorithms learn, we must train them. And for training, we need data, a LOT of data. This data needs to be clean & pre-processed in neat columns and rows, making it easier for these algorithms to ingest. This is a challenge, as most data from the healthcare sector is chaotic and noisy. All AI algorithms are useless if we cannot provide clean data, and as a first step, we clinicians need to adopt a system where we can digitise and store our data in a way which can be fed into the algorithm. This data should (ideally) be collected prospectively, along with our routine clinical work. There will be inertia initially (and if you have juniors working with you, perhaps resistance too!), but it quickly becomes a part of the usual routine once we get used to it and will yield future rewards.
Healthcare DataThe AI Techniques: ML, DL and Natural Language Processing (NLP)
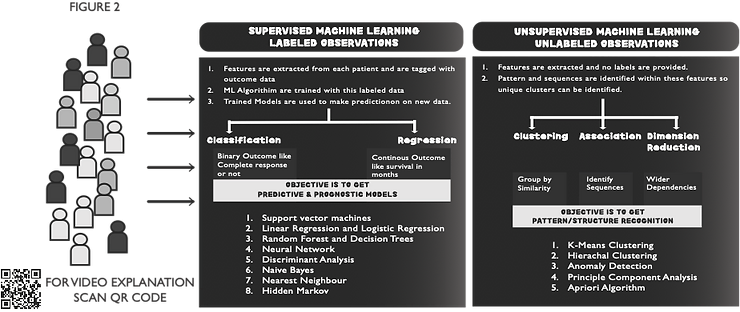
ML comprises various data analysis algorithms that help extract valuable features from complex data and predict the outcome of interest. It can be divided into Unsupervised ML (UML) or Supervised ML (SML) (Figure 2). UML is usually used to extract features (clinical, imaging, genetic, and many other features) without focusing on the outcome. The goal is to discover features which can divide the populations into unique clusters (unbiased by our clinical judgement) and could demonstrate a difference in clinical outcomes. Once we have these features, we use them to define a predictive and prognostic model for our outcome of interest by using SML methods(1).
NLP
ML algorithms can use imaging and genetic data with relative ease compared to clinical data. Although clinical data is human-readable, it is tough for machines to comprehend it until we pre-process it manually. If we have a massive pile of clinical data, manual pre-processing becomes arduous, and it is often impossible to decipher helpful information. In this situation, NLP significantly extracts valuable information from the narrative text to assist clinical decision-making. For example, it can read Chest X-ray reports and alert us about antibiotic use (2) or automatically alert us if there are some issues with laboratory results (3).
Deep Learning: a more advanced ML technique
A neural network with a single layer of nodes (each node is a mathematical linear regression expression) to capture non-linear patterns in data is classically used as one of the SML methodologies. Deep learning is the broader and deeper extension of this classic neural network. In most cases, deep learning is used in conjunction with complex medical images with a vast amount of numerical data in each image. One commonly used algorithm in this context is Convolutional Neural Network (CNN).
Medical images are taken as input, and relevant, helpful information is extracted from those images. It is correlated with the clinical outcome of interest, and finally, we have a fully trained model which can predict the outcome based on medical images. This whole feature extraction process from medical images and model building is fully automated without human intervention. This is also one of the drawbacks, as this entire process works as a ‘Blackbox’, where we do not have any access to the logic of decision-making.
However, recent advances have somewhat weakened this criticism by plotting the weights of different layers of CNN into the image and thus highlighting the area most helpful in decision-making. This allows us to understand and confirm the biological basis of decision-making. For example, in lung cancer, a pre-treatment CT scan can be used to predict the survival outcome (4, 5) and post-SBRT risk of relapse, as well as radiation pneumonitis (6, 7).
AI: Clinical Application
The clinical application of these AI methodologies is vast and can be applied at any specific point in the cancer care continuum, from early detection to outcome prediction.
1. Early detection & diagnosis: In breast cancer (using a Mammogram) (8) and Lung cancer (using Low Dose Screening CT scan) (9), suspicious lesions are automatically annotated in the images and classified as malignant or benign so that further diagnostic interventions can be done.
2. Treatment: Clinical decision-making is a field that is being studied extensively. We do not have any reliable model that can replace an experienced clinician in making treatment decisions today. We may never have this kind of scenario in the future, either. However, we can aim to build AI models that can assist clinicians in their decision-making to smoothen the clinical workflow and thus improve the patient’s clinical outcome. Along similar lines, we are developing a CNN model based on Chest X-Ray images (Figure 3A) to predict the suitability of left-sided breast cancer patients who require adjuvant RT for cardiac sparing (using Deep Inspiration Breath Hold) techniques(10).
3. Outcome prediction and prognosis evaluation: This field is extensively used. Classifying Breast cancer or Lung cancer based on relevant medical images into the distinct molecular group is one part of it and predicting pathological response to neoadjuvant treatment strategies is another part (4, 5). Our group is working on a project where we aim to predict a complete pathological response after surgery in patients with oesophageal cancer (Figure 3B) undergoing neoadjuvant concurrent chemo-radiotherapy(11).

Summary
The application of Artificial Intelligence in all oncological specialities across every aspect of the cancer care continuum is the future and cannot be ignored. However, to harness its true potential, we need to understand its capabilities and limitations and must prepare to be AI-ready by adopting modern clinical data recording practices. The promise of AI will be delivered when we have models customised to our needs, assisting us in caring for our patients.
References:
- Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-43.
- Fiszman M, Chapman WW, Aronsky D, Evans RS, Haug PJ. Automatic detection of acute bacterial pneumonia from chest X-ray reports. J Am Med Inform Assoc. 2000;7(6):593-604.
- Miller TP, Li Y, Getz KD, Dudley J, Burrows E, Pennington J, et al. Using electronic medical record data to report laboratory adverse events. Br J Haematol. 2017;177(2):283-6.
- Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006.
- Hosny A, Parmar C, Coroller TP, Grossmann P, Zeleznik R, Kumar A, et al. Deep learning for lung cancer prognostication: A retrospective multi-cohort radiomics study. PLoS Med. 2018;15(11):e1002711.
- Baek S, He Y, Allen BG, Buatti JM, Smith BJ, Tong L, et al. Deep segmentation networks predict survival of non-small cell lung cancer. Sci Rep. 2019;9(1):17286.
- Adachi T, Nakamura M, Shintani T, Mitsuyoshi T, Kakino R, Ogata T, et al. Multi-institutional dose-segmented dosiomic analysis for predicting radiation pneumonitis after lung stereotactic body radiation therapy. Med Phys. 2021;48(4):1781-91.
- Mao N, Yin P, Wang Q, Liu M, Dong J, Zhang X, et al. Added Value of Radiomics on Mammography for Breast Cancer Diagnosis: A Feasibility Study. J Am Coll Radiol. 2018.
- Binczyk F, Prazuch W, Bozek P, Polanska J. Radiomics and artificial intelligence in lung cancer screening. Transl Lung Cancer Res. 2021;10(2):1186-99.
- Chufal KS, Ahmad I, Sharief MI, Dwivedi A, Bajpai R, Miller AA, et al. Convolutional Neural Network to predict Deep Inspiration Breath Hold eligibility using Chest X-Ray. Radiotherapy & Oncology. 2021;161:S560-S1.
- Chufal KS, Ahmad I, Dwivedi A, Bajpai R, Miller AA, Chowdhary RL, et al. Deep learning using Pre-NACRT imaging can predict pathological response in esophageal cancer. Radiotherapy & Oncology. 2021;161:S1530 – S1.

